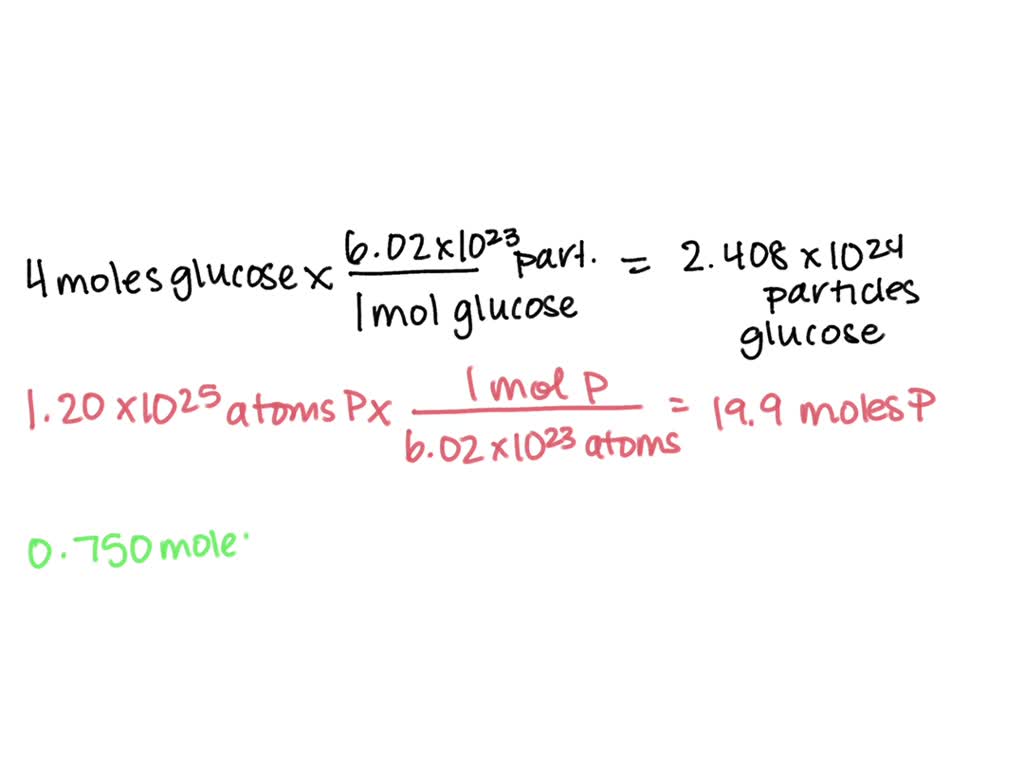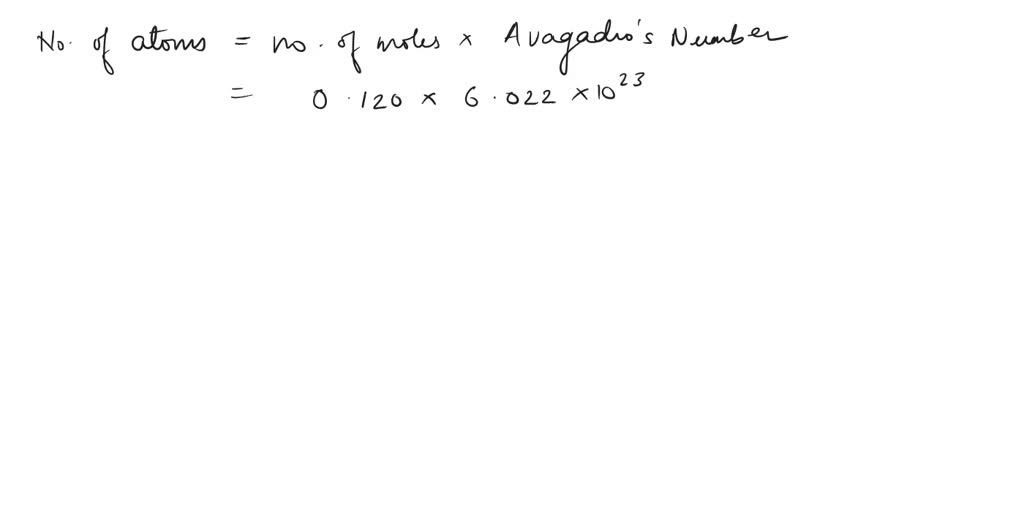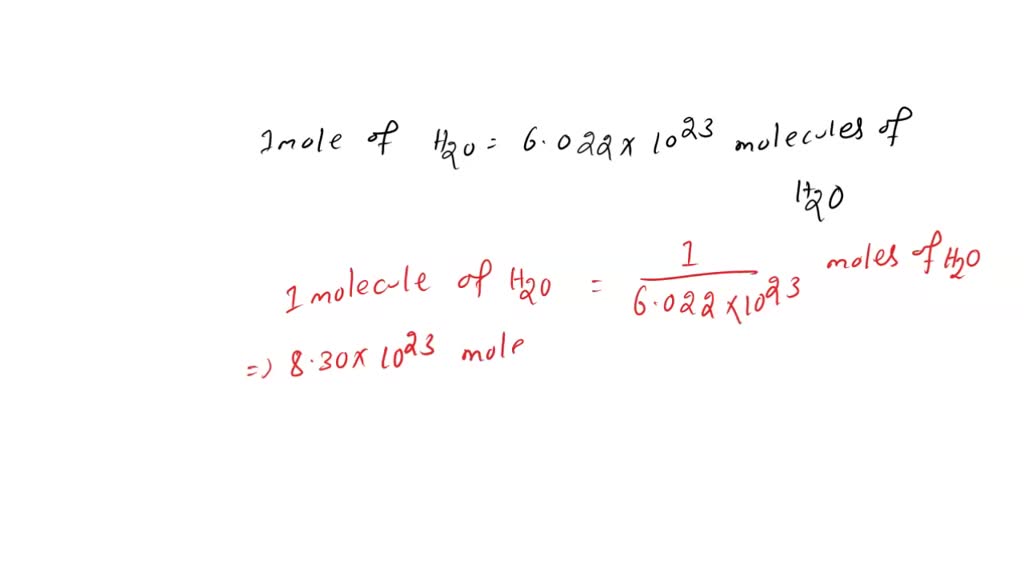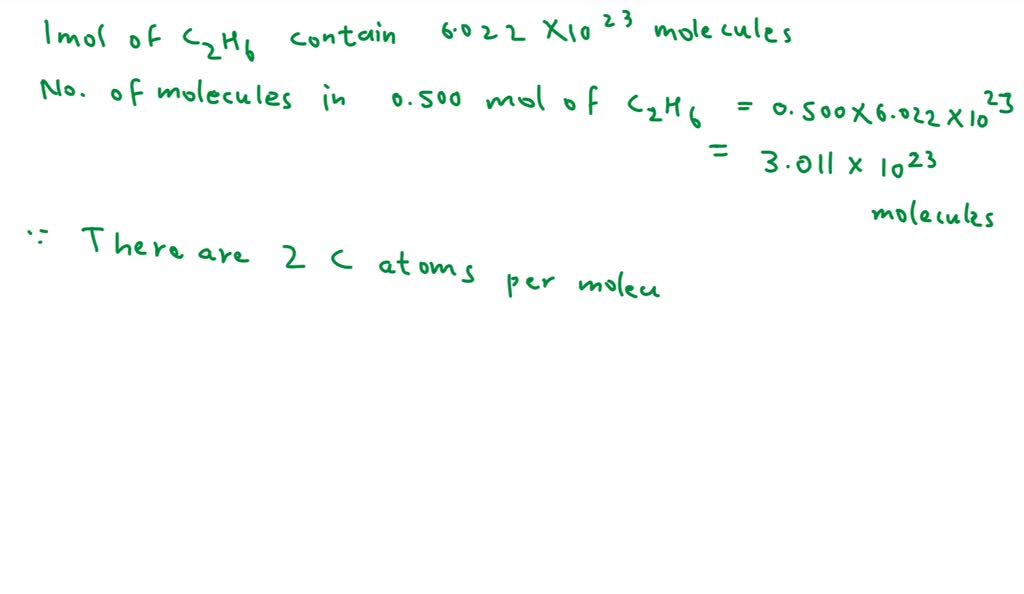Convert 3 01 X 1023 Molecules Of C2H6 To Moles - To convert molecules to moles, we use avogadro's number, which is approximately 6.022 x 10^23 molecules per mole. Calculate the moles of c 2h 6 in 3.75× 1023 molecules of c 2h 6. 1 mole contains 6.022× 1023 number of molecules. Use this simple science molecules to moles calculator to calculate mole. To convert molecules of a substance to moles, you need to use avogadro's number, which is approximately 6.022 x 10^23. (3.01 × 10²³ molecules) / (6.02214076 × 10^23 molecules/mol) = 0.50 moles. Convert 3.01×10 23 molecules of c 2 h 6 to moles. One mole of any substance contains 6.02×10 23 atoms/molecules. Online molecules to moles calculation. So, there are approximately 0.50 moles of.
1 mole contains 6.022× 1023 number of molecules. Online molecules to moles calculation. To convert molecules of a substance to moles, you need to use avogadro's number, which is approximately 6.022 x 10^23. To convert molecules to moles, we use avogadro's number, which is approximately 6.022 x 10^23 molecules per mole. One mole of any substance contains 6.02×10 23 atoms/molecules. So, there are approximately 0.50 moles of. Convert 3.01×10 23 molecules of c 2 h 6 to moles. (3.01 × 10²³ molecules) / (6.02214076 × 10^23 molecules/mol) = 0.50 moles. Use this simple science molecules to moles calculator to calculate mole. Calculate the moles of c 2h 6 in 3.75× 1023 molecules of c 2h 6.
So, there are approximately 0.50 moles of. Calculate the moles of c 2h 6 in 3.75× 1023 molecules of c 2h 6. (3.01 × 10²³ molecules) / (6.02214076 × 10^23 molecules/mol) = 0.50 moles. To convert molecules to moles, we use avogadro's number, which is approximately 6.022 x 10^23 molecules per mole. Convert 3.01×10 23 molecules of c 2 h 6 to moles. 1 mole contains 6.022× 1023 number of molecules. Use this simple science molecules to moles calculator to calculate mole. One mole of any substance contains 6.02×10 23 atoms/molecules. To convert molecules of a substance to moles, you need to use avogadro's number, which is approximately 6.022 x 10^23. Online molecules to moles calculation.
Solved How many moles of CO2 are produced when 3.01 x 1023
To convert molecules to moles, we use avogadro's number, which is approximately 6.022 x 10^23 molecules per mole. So, there are approximately 0.50 moles of. Use this simple science molecules to moles calculator to calculate mole. To convert molecules of a substance to moles, you need to use avogadro's number, which is approximately 6.022 x 10^23. Online molecules to moles.
SOLVED 1 mol = 6.02 x 1023 particles (atoms or ions or molecules etc
1 mole contains 6.022× 1023 number of molecules. To convert molecules to moles, we use avogadro's number, which is approximately 6.022 x 10^23 molecules per mole. One mole of any substance contains 6.02×10 23 atoms/molecules. (3.01 × 10²³ molecules) / (6.02214076 × 10^23 molecules/mol) = 0.50 moles. Use this simple science molecules to moles calculator to calculate mole.
SOLVED Calculate the number of atoms in 0.120 moles of Na. Calculate
(3.01 × 10²³ molecules) / (6.02214076 × 10^23 molecules/mol) = 0.50 moles. Use this simple science molecules to moles calculator to calculate mole. So, there are approximately 0.50 moles of. Convert 3.01×10 23 molecules of c 2 h 6 to moles. Online molecules to moles calculation.
SOLVED Calculate the number of moles of C2H6 (nC2H6) in 6.75 x 10^23
Convert 3.01×10 23 molecules of c 2 h 6 to moles. 1 mole contains 6.022× 1023 number of molecules. (3.01 × 10²³ molecules) / (6.02214076 × 10^23 molecules/mol) = 0.50 moles. Use this simple science molecules to moles calculator to calculate mole. To convert molecules of a substance to moles, you need to use avogadro's number, which is approximately 6.022.
Solved How many moles of CO2 are produced when 3.01 x 1023
(3.01 × 10²³ molecules) / (6.02214076 × 10^23 molecules/mol) = 0.50 moles. Calculate the moles of c 2h 6 in 3.75× 1023 molecules of c 2h 6. One mole of any substance contains 6.02×10 23 atoms/molecules. To convert molecules of a substance to moles, you need to use avogadro's number, which is approximately 6.022 x 10^23. Use this simple science.
SOLVED B How nHY 1 '24 1 moles 8 m 2 molecules water molecules 3 are
(3.01 × 10²³ molecules) / (6.02214076 × 10^23 molecules/mol) = 0.50 moles. Online molecules to moles calculation. Use this simple science molecules to moles calculator to calculate mole. 1 mole contains 6.022× 1023 number of molecules. Convert 3.01×10 23 molecules of c 2 h 6 to moles.
How many moles of carbon atoms are there in 0.500 mole of C2H6? 4.00
To convert molecules of a substance to moles, you need to use avogadro's number, which is approximately 6.022 x 10^23. So, there are approximately 0.50 moles of. Use this simple science molecules to moles calculator to calculate mole. Convert 3.01×10 23 molecules of c 2 h 6 to moles. To convert molecules to moles, we use avogadro's number, which is.
[Solved] How many moles are in 2.58 x 1024molecules of carbon dioxide
(3.01 × 10²³ molecules) / (6.02214076 × 10^23 molecules/mol) = 0.50 moles. 1 mole contains 6.022× 1023 number of molecules. One mole of any substance contains 6.02×10 23 atoms/molecules. Use this simple science molecules to moles calculator to calculate mole. Online molecules to moles calculation.
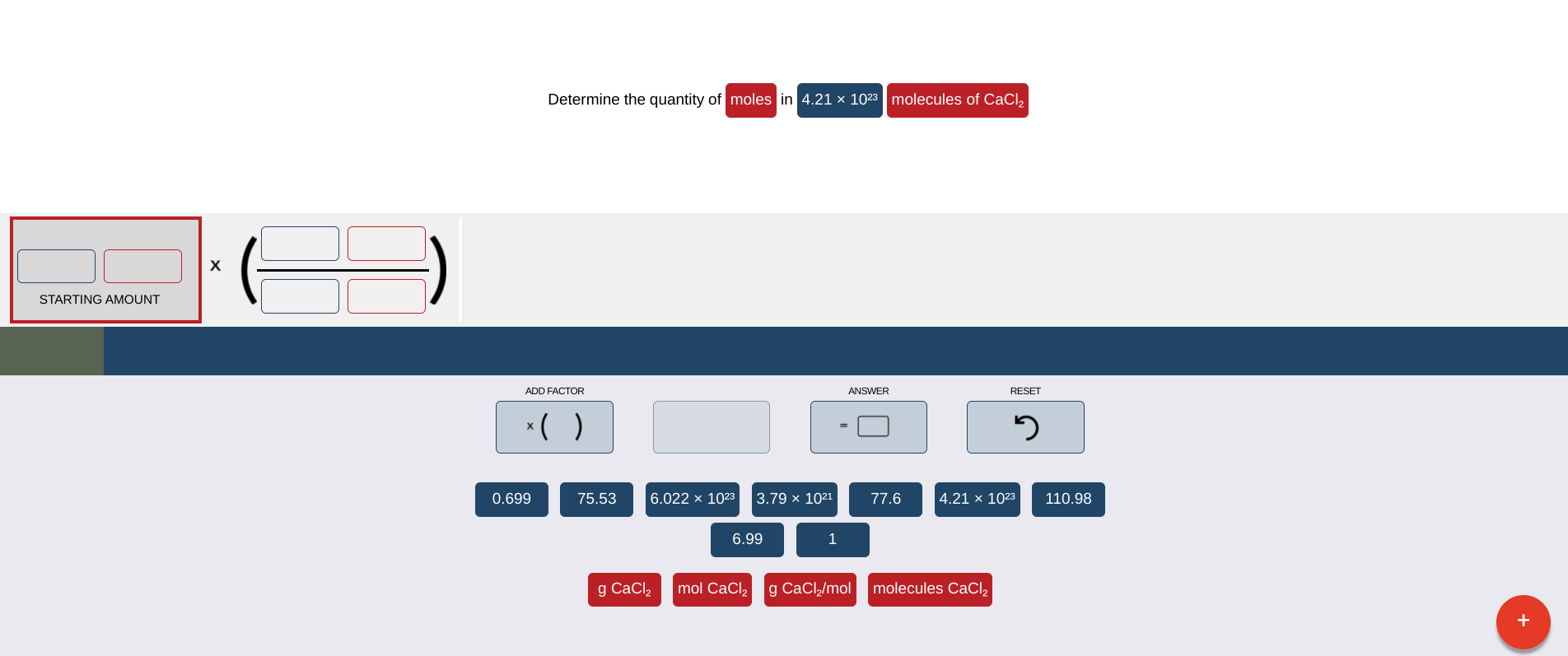
Solved Determine the quantity of moles in 4.21 x 1023
So, there are approximately 0.50 moles of. Use this simple science molecules to moles calculator to calculate mole. One mole of any substance contains 6.02×10 23 atoms/molecules. (3.01 × 10²³ molecules) / (6.02214076 × 10^23 molecules/mol) = 0.50 moles. To convert molecules to moles, we use avogadro's number, which is approximately 6.022 x 10^23 molecules per mole.
Molecules To Moles
So, there are approximately 0.50 moles of. One mole of any substance contains 6.02×10 23 atoms/molecules. (3.01 × 10²³ molecules) / (6.02214076 × 10^23 molecules/mol) = 0.50 moles. Calculate the moles of c 2h 6 in 3.75× 1023 molecules of c 2h 6. To convert molecules to moles, we use avogadro's number, which is approximately 6.022 x 10^23 molecules per.
Online Molecules To Moles Calculation.
One mole of any substance contains 6.02×10 23 atoms/molecules. (3.01 × 10²³ molecules) / (6.02214076 × 10^23 molecules/mol) = 0.50 moles. Use this simple science molecules to moles calculator to calculate mole. To convert molecules of a substance to moles, you need to use avogadro's number, which is approximately 6.022 x 10^23.
So, There Are Approximately 0.50 Moles Of.
Calculate the moles of c 2h 6 in 3.75× 1023 molecules of c 2h 6. To convert molecules to moles, we use avogadro's number, which is approximately 6.022 x 10^23 molecules per mole. Convert 3.01×10 23 molecules of c 2 h 6 to moles. 1 mole contains 6.022× 1023 number of molecules.