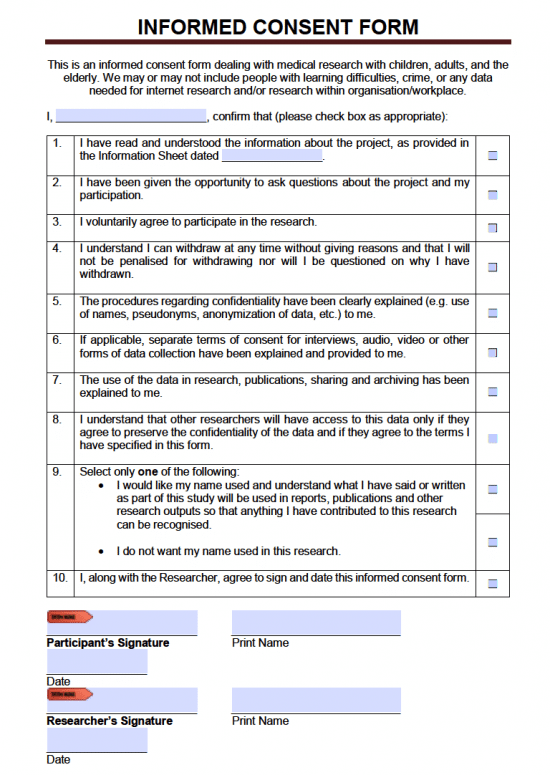
Consent Form Template For Research - Before research begins, it is important to first obtain participant’s consent on the basis of their full and proven understanding of what the. This template can be used by researchers to gain informed consent to conduct research that collects data from people using questionnaires,. Please note that these are templates developed by the who erc to assist the principal investigator in the design of their informed. Required only when applicable to your. Please note that this is a template developed by the research ethics review office to assist research proponents in the design of their. A collection of informed consent, assent, and debriefing templates that can be used for your human participant research study. Identifies consent elements and information required for all st. A research informed consent form is used to inform participants in a research study of how the research will be conducted,. See irb guidance on children and minors in research for information about the documentation needed for consenting children and parents, and. Entifies consent elements or info.
Entifies consent elements or info. Please note that this is a template developed by the research ethics review office to assist research proponents in the design of their. Identifies consent elements and information required for all st. Before research begins, it is important to first obtain participant’s consent on the basis of their full and proven understanding of what the. Please note that these are templates developed by the who erc to assist the principal investigator in the design of their informed. A research informed consent form is used to inform participants in a research study of how the research will be conducted,. This template can be used by researchers to gain informed consent to conduct research that collects data from people using questionnaires,. A collection of informed consent, assent, and debriefing templates that can be used for your human participant research study. See irb guidance on children and minors in research for information about the documentation needed for consenting children and parents, and. Required only when applicable to your.
See irb guidance on children and minors in research for information about the documentation needed for consenting children and parents, and. Before research begins, it is important to first obtain participant’s consent on the basis of their full and proven understanding of what the. Identifies consent elements and information required for all st. Entifies consent elements or info. A collection of informed consent, assent, and debriefing templates that can be used for your human participant research study. A research informed consent form is used to inform participants in a research study of how the research will be conducted,. Required only when applicable to your. This template can be used by researchers to gain informed consent to conduct research that collects data from people using questionnaires,. Please note that these are templates developed by the who erc to assist the principal investigator in the design of their informed. Please note that this is a template developed by the research ethics review office to assist research proponents in the design of their.
Free Research Informed Consent Form PDF Word eForms
A collection of informed consent, assent, and debriefing templates that can be used for your human participant research study. Please note that this is a template developed by the research ethics review office to assist research proponents in the design of their. Entifies consent elements or info. Before research begins, it is important to first obtain participant’s consent on the.
Data Consent Form Template
A research informed consent form is used to inform participants in a research study of how the research will be conducted,. This template can be used by researchers to gain informed consent to conduct research that collects data from people using questionnaires,. Required only when applicable to your. Entifies consent elements or info. Please note that this is a template.
Consent Form Template For Research Study SampleTemplatess
Please note that this is a template developed by the research ethics review office to assist research proponents in the design of their. This template can be used by researchers to gain informed consent to conduct research that collects data from people using questionnaires,. Identifies consent elements and information required for all st. A collection of informed consent, assent, and.
Informed Consent Form Template For Research DocTemplates
Please note that this is a template developed by the research ethics review office to assist research proponents in the design of their. Please note that these are templates developed by the who erc to assist the principal investigator in the design of their informed. A research informed consent form is used to inform participants in a research study of.
FREE 12+ Research Consent Form Samples, PDF, MS Word, Google Docs
A collection of informed consent, assent, and debriefing templates that can be used for your human participant research study. See irb guidance on children and minors in research for information about the documentation needed for consenting children and parents, and. Before research begins, it is important to first obtain participant’s consent on the basis of their full and proven understanding.
Research Consent Forms Template Flyer Template
This template can be used by researchers to gain informed consent to conduct research that collects data from people using questionnaires,. Please note that these are templates developed by the who erc to assist the principal investigator in the design of their informed. A research informed consent form is used to inform participants in a research study of how the.
Informed Consent Form Template for clinical trials
Please note that these are templates developed by the who erc to assist the principal investigator in the design of their informed. See irb guidance on children and minors in research for information about the documentation needed for consenting children and parents, and. Please note that this is a template developed by the research ethics review office to assist research.
FREE 6+ Research Consent Forms in PDF MS Word
A collection of informed consent, assent, and debriefing templates that can be used for your human participant research study. Please note that this is a template developed by the research ethics review office to assist research proponents in the design of their. Please note that these are templates developed by the who erc to assist the principal investigator in the.
Research Consent Form Fill Out, Sign Online and Download PDF
See irb guidance on children and minors in research for information about the documentation needed for consenting children and parents, and. Entifies consent elements or info. Before research begins, it is important to first obtain participant’s consent on the basis of their full and proven understanding of what the. This template can be used by researchers to gain informed consent.
Consent Form For Research Word PDF Google Docs
Please note that this is a template developed by the research ethics review office to assist research proponents in the design of their. A collection of informed consent, assent, and debriefing templates that can be used for your human participant research study. Entifies consent elements or info. Before research begins, it is important to first obtain participant’s consent on the.
This Template Can Be Used By Researchers To Gain Informed Consent To Conduct Research That Collects Data From People Using Questionnaires,.
A research informed consent form is used to inform participants in a research study of how the research will be conducted,. Please note that these are templates developed by the who erc to assist the principal investigator in the design of their informed. A collection of informed consent, assent, and debriefing templates that can be used for your human participant research study. Before research begins, it is important to first obtain participant’s consent on the basis of their full and proven understanding of what the.
Required Only When Applicable To Your.
Entifies consent elements or info. See irb guidance on children and minors in research for information about the documentation needed for consenting children and parents, and. Please note that this is a template developed by the research ethics review office to assist research proponents in the design of their. Identifies consent elements and information required for all st.